THE NON-INVASIVE MOVEMENT:
AVOID THE RISKS OF CUTTING & UNNECESSARY BIOPSIES
When it comes to finding abnormalities in a patient exam, many conventional-minded doctors tend to tread on the side of caution... but usually at YOUR expense! They find an unusual spot that appears questionable and their first reaction is to cut it out and send it to the lab for a BIOPSY. As with all invasive surgical procedures (however large or small) carries risks including bleeding, infections, post-surgical scars and potential damage to nearby tissues and organs.
The year is 2019- the era of non-invasive technologies where identifying what's under the skin no longer needs to be about cutting into it. The age of robotics, artificial intelligence (AI), highly developed laser applications and advanced sonic diagnostic protocols are all fast replacing the age-old scalpel as part of risk reduction, time/cost advantages and increased performance in the world of clinical diagnostics and medical treatment.
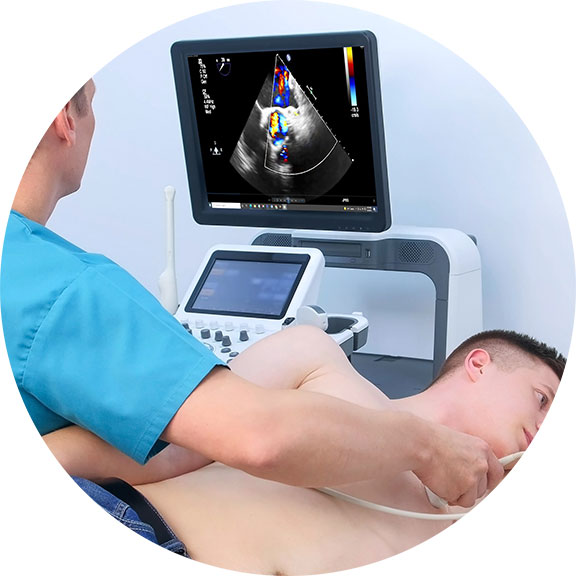
Imaging technologies like the 4D Doppler Ultrasound™ can accurately and successfully scan, study and fully diagnose any skin anomalies and sharply view what's going on underneath. Derm imaging specialists stand on the side of innovation as they confidently rely on the most current devices to deliver the most accurate readings while bringing significant reduction to patient stress under a scan- that takes mere minutes!
"Before & After" Studies
The most sensible and logical way to identify the results of any treatment is by tracking the body's response to it. Controlled testing must show the patient's condition PRE and POST effects, where true data-finding is collecting the necessary EVIDENCE of its claims. The investigator can pull a significant amount of data from this form of oberservational testing and recording: including stage-by-stage bodily response to future projections of possible side effects. Recording of any and all psysiological response means the researchers are counting on the patient's body to tell us what it is undergoing during the testing phase. To prevent mis-reading and erroneous reports, trials tend to work with a large number of test patients (commonly 50-100) and may also employ redundancies like undergoing multiple testing protocols for a second or even third opinion. To capture the benefits of a BEFORE AND AFTER review, Imaging is often used as a standard screening solution for the response of most of the major organs.
|
"SEEING IS BELIEVING":
Advantages of Imaging in Research Studies
 QUANTIFIABLE DETECTION OF THERAPY RESPONSE QUANTIFIABLE DETECTION OF THERAPY RESPONSE
MEDICAL IMAGING refers to the set of FDA approved modalities that are used to view the human body in order to diagnose and monitor medical conditions or the response of its treatments. The types of common modalities include X-rays, MRI (magnetic resonance imaging), Ultrasound, CT scan (computed tomography scan) and Nuclear medicine imaging including Positron Emission Tomography (PET) [1][2]
Ultrasound imaging (sonography) uses high-frequency sound waves to view inside the body. Because ultrasound images are captured in real-time, they can also show movement of the body's internal organs as well as blood flowing through the blood vessels. Unlike X-ray imaging, Ultrasound uses NO ionizing radiation exposure whatsoever.[3] This modality (sometimes referred to as "the orthopedic stethoscope"), offers easy access, portability, affordability and real-time monitoring and is safe for all patients, including those with cardiac pacemakers and metal implants, without any contraindications.
TRACKING THERAPEUTIC EFFECTS THROUGH BLOOD FLOW REACTIONS
To date, the ultrasound's ability to evaluate abnormalities within the soft tissue such as cysts, tumors and inflammation is used to help identify an expanded set of pathologies in the body. Since the early 1970's, Dr. Robert L. Bard (NYC Cancer Radiologist) has used diagnostic imaging for pre and post procedural guidance, and diagnostic care (screening and monitoring) of his patients. Dr. Bard is also recognized for his use of ultrasound in pharmaceutical research and clinical trials, where his leadership in analytical interpretation is sought after worldwide for identifying markers and therapeutic efficacy.
Throughout his career, Dr. Bard has employed this imaging strategy to detect, track and confirm the body's reaction to a variety of therapeutic interventions. He has conducted medical center based double-blinded, corporate sponsored and private studies reviewing the effects of injectable therapies (PRP, Stem Cell therapies, etc) as well as non-invasive therapeutic interventions in studies of neuro-stimulation, electrostimulation and electromagnetic field treatments. His approach involves the comparative study of measurable scanning data or quantitative ultrasound (QUS) which aims at recording interactions between the behavior and activity of biological tissue microstructure and ultrasound waves [5][6]. From a time-based comparative study of the treated area (before and after studies), Dr. Bard applies the use of blood flow detection technology or hemodynamic data gathering protocols, document specific objective and quantifiable biological responses to therapeutic treatments.
Ref:
1) Medical Imaging/FDA: https://www.fda.gov/radiation-emitting-products/radiation-emitting-products-and-procedures/medical-imaging#:~:text=Medical%20imaging%20refers%20to%20several,monitor%2C%20or%20treat%20medical%20conditions.
2) https://sonosimaging.com/press-corner/understanding-the-different-types-of-imaging/
3) https://www.fda.gov/radiation-emitting-products/medical-imaging/ultrasound-imaging
4) Diagnostic imaging to detect and evaluate response to therapy in bone metastases from prostate cancer: current modalities and new horizons https://pubmed.ncbi.nlm.nih.gov/26956538/ |
|
IRB-BASED VALIDATION STUDY / VISUAL AND QUANTIFIABLE REPORTING OF NON-INVASIVE THERAPEUTICS THROUGH MEDICAL IMAGING PROTOCOLS
By the year 2000, the vast majority of non‐invasive wellness devices on the public market have deep rooted themselves into the WELLNESS and FUNCTIONAL communities, leading to the sales of ‘invisible treatments’. These devices employ one of a number of technology based modalities including: pulsed electromagnetic frequency, biofeedback, shockwave sonic pulse as well as light‐related energy therapies like cold laser, blue light and infrared just to name a few.
The concept behind ‘MEDICAL VALIDATION’ underscores the complex commitment of “ensuring that the medical device being manufactured will consistently provide the intended benefits for its use condition. Clinical validation is usually done through a series of tests and inspections.” [1] To conduct this medical validation officially can be offered through various protocols, an Institutional Review Board approved by the HHS and FDA regulations. One protocol that can be available for consideration by the IRB panel is the use of medical grade 3D Doppler Ultrasound imaging. The use of medical imaging technologies like an ultrasound alongside the interpretation of a certified clinical radiologist may offer biometric assessment and analyses of all scanned readings and the collection of ample data to confirm or validate a health device’s effects on the body as marketed.
 |
PERFORMANCE TESTING & VALIDATION PROGRAM MAY CHALLENGE OR SUPPORT PUBLIC CLAIMS
Especially when a product is promoted as a health‐related solution, testing is an important and highly critical step from a legal standpoint. This step is directly involved in a developer’s statements and claims about the device. Before any device is to be promoted for a specific pathology, a series of formal performance exams must be conducted to suit regulatory acceptance by an outside agency or clinical team approved by a federally backed review board. Considerations for validation include: range of physiological effects, warnings about the possible hazards, physical risk to the user in regular use, adverse reactions (potential side effects)‐ and all these elements are from data acquired during a validation study [2][3]. To confirm (or validate) that a product is effective within the statements and claims that it provides the public, the manufacturer would need to invest in an exclusive and dedicated lab or agency that is poised to perform this level of testing. Said lab must acquire enough data (according to the IRB board) to supports the aspired claims. If/when enough data has proven the hypothesis true, this would add to the product’s marketing credence as well as confirms the device’s ability as a health or wellness product. Any other type of observational testing outside of the authority of an IRB is considered ANECDOTAL and must be established as such‐ which holds no credence as an industrywide claim whatsoever. |
Recent & Innovative PROJECTS
Within the past decade, the world of medicine has witnessed (and achieved) a significant amount of success with STEM CELL therapies managing a wide range of disorders. There are 2 main types of stem cell transplant processes- AUTOLOGOUS (source of harvested cells are the same as the recipient) and ALLOGENIC (sourced from a matching donor). Concentrates of regenerative cells can be extracted from bone marrow, unbillical cord (newborns) or fat (adipose) cells & bloodstream. Patients are (now) recognized to receive treatments for a growing list of isses such as ORTHOPEDIC damage (bone and joint injuries) and AUTO-IMMUME DISORDERS (lupus, arthritis, diabetes ). NEUROLOGICAL ISSUES are also now being addressed by stem cell treatments whereby research and testing for treatment efficacy relating to the Nervous System is also in full swing within the clinical community.
Dr. Bard's 4D Doppler
capabilities have become the preferred non-invasive solution with research programs to seek out treatment testing or application performance tracking for this exact type of exploratory testing. Dr. Bard has the capacity to identify and track prestroke vasospasms, any increase in intracranial pressure, brain aneurysms
and many degenerative disorders. (See related article) |
Early collaboration with technological FRONTIERSHIP
 |
In 1976, during Dr. Bard's days as a young radiologist, he was approached by Dr. Henry Leis Jr., the pioneer doctor who wrote the very first text on breast cancer and developed mammography 18- a means of early diagnosis and instrumental in the use of many of the less invasive procedures used in the treatment of breast cancer today. He confessed with great concern that he had all these patients with lumpy or cystic breasts developing tumors that he could clearly feel but the mammogram kept missing it. Seeking Dr. Bard's help through sonogram technology, they worked on his patients together and the sonogram clearly identified and quickly diagnosed a mass as either a cancer or a benign cyst, in a dense, lumpy breast. Since then, the sonogram became incorporated in high-risk patients’ regimen every six months religiously because it finds tumors while they're small and “lumpectomy” surgery is curative if the mass is less than 1 cm. This is alongside doing mammograms once a year in women over 50 or unless they have a history of cancer- at which case, we do it starting at age 45. |
|
|

